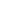Iron, oxygen, water, these three elements combined together to form a "primary battery", and this corrosion is also known as "electrochemical corrosion". The essence of the redox reaction, and the underlying cause is what we often call "electron migration".

When we understand the theory of redox, we can quantitatively explain the mechanism of corrosion.
Iron atoms in the action of oxygen, will lose electrons to produce iron oxide.
Water acts as the electrolyte necessary for electron migration during the reaction.
Nature's acidic and basic substances act as catalysts to accelerate the reaction.
Corrosion of photovoltaic mounts.
For the design of PV mounts, we consider the effects of corrosion from three main sources, they are.
▵ Atmospheric corrosion: Exposed in the atmosphere of the upper solar panel bracket structure, will interact with the oxygen in the air and water to produce corrosion, this corrosion is the most common, but also the most easily perceived.
▵ Soil corrosion: buried deep in the ground of the PV steel pile foundation, in the soil under the action of various substances, will carry out more complex chemical reaction, thus corrosion. This corrosion is difficult to detect because it is in the soil for a long time.
▵ Contact corrosion: between different metals, due to the difference in potential, will occur the gain and loss of electrons, thus forming electrochemical corrosion, this corrosion is often ignored by the designer.

Methods of solar bracket corrosion.
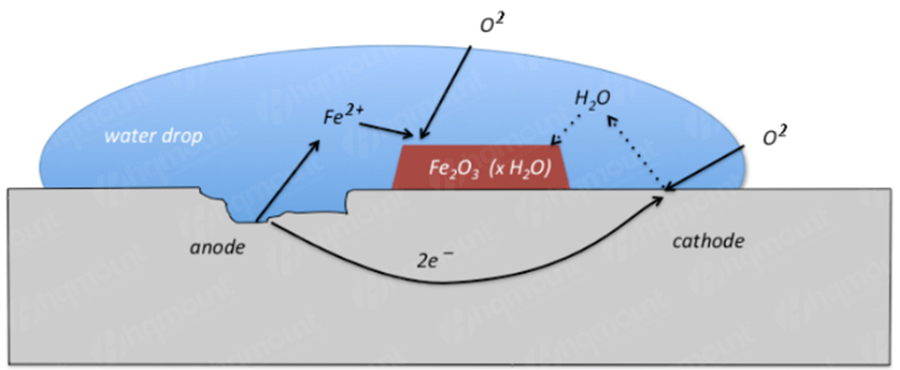
In order to slow down or even avoid steel corrosion, a common method in the industry is to "galvanize" the steel surface. The standard potential of zinc at 25°C is -0.76V, which is lower than that of iron at -0.45V, so zinc is more "active" than iron. Zinc and iron in a humid environment to form a "primary battery", the same as all the principles of electrochemical corrosion, the primary battery combination is: "sacrificial anode (zinc), the protection of the cathode (iron)" the lower the rank, the easier to lose electrons.
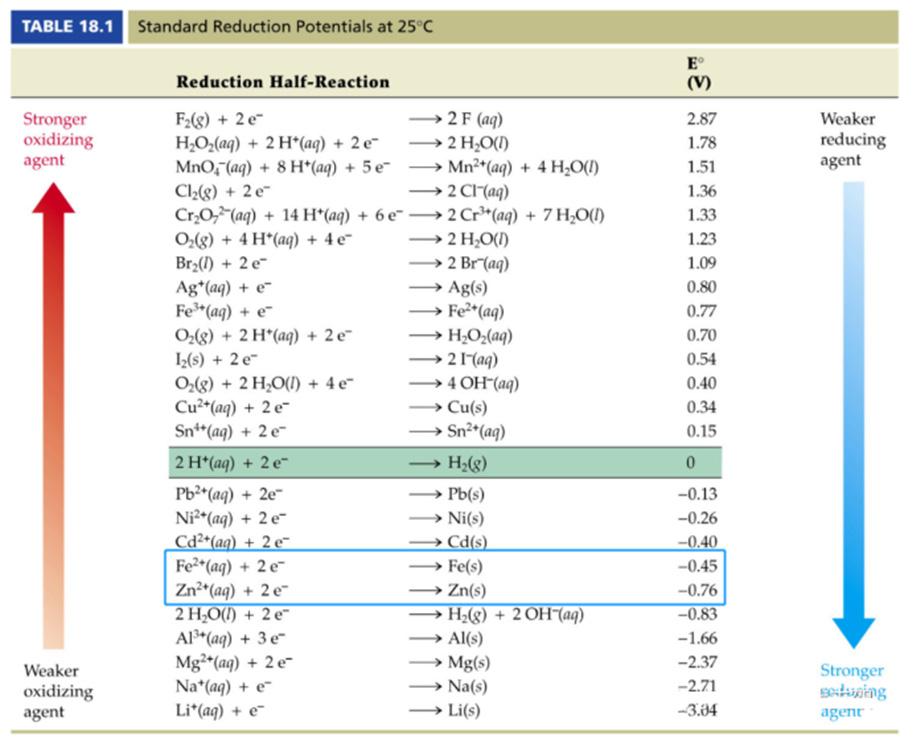
The common galvanizing processes for solar mounting brackets are: hot-dip galvanizing, pre-galvanizing, and electro-galvanizing (cold galvanizing). Due to the primary cell effect of zinc and iron, even minor damage to the galvanized layer during transportation or installation does not affect the corrosion protection of the galvanized layer. Of course, extra care is required for deeper damage, and these areas need to be treated with zinc replenishment.
▵ The primary cell formed by zinc, iron and oxygen in a humid environment will not affect the corrosion resistance of the galvanized layer even if it is slightly scratched. In addition, zinc reacts with air and water to produce a new product: alkaline zinc carbonate, which has a dense surface and does not dissolve easily in water, and can well isolate air from iron, thus forming another layer of "physical protection", further slowing down the corrosion of steel.
Xiamen HQ Mount has rich experience in designing as well as manufacturing photovoltaic brackets, and in the face of solar bracket corrosion problems, our photovoltaic supports have an average service life of more than 25 years, if you want to know more information about solar brackets, welcome to contact us!
 86 05926252889
86 05926252889 allie@hqmount.com
allie@hqmount.com